19 Jan 2024| By: Abdullah Hussein
Asphaltenes are the heaviest components of crude oil. They are dark in color and are usually referred to as "the cholesterol of oil" . Like the bad cholesterol in the blood stream, asphaltenes are well known to cause several operational issue, such as:
- Formation damage
- Rock wettability change
- Stabilizing emulsions and interfering with phase separation
- Flow restrictions and blockages
- Equipment damage
- Inducing wax gelation and increasing oil viscosity
- Interfering with produced water treatment chemicals and other production chemicals.
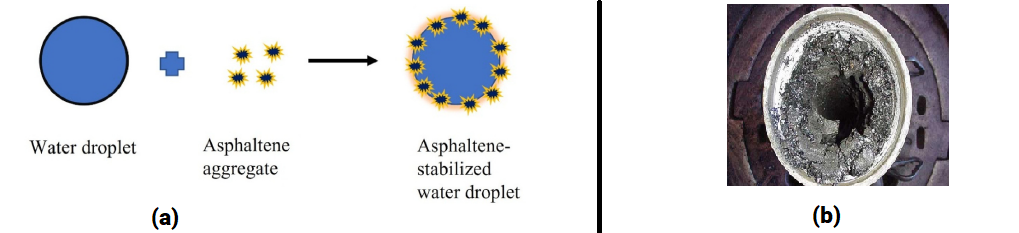
Fig.1: Examples of asphaltenes problems: (a) Emulsion stabilization by asphaltenes (source Yonguep, et al. (2022) Petroleum Research, https://doi.org/10.1016/j.ptlrs.2022.01.007) (b) Asphaltenes deposits (courtesy of baker Hughes)
In the literature, asphaltenes are usually defined as a solubility class: alkane insoluble and toluene soluble fraction of crude oil. Asphaltenes composition usually comprise polyaromatic cores, aliphatic chains, heteroatoms (N, O, S), and trace metals (Ni, V, Fe). Two main structures are currently used to represent asphaltenes: the continental and the archipelago structures, as shown in Fig. 2.
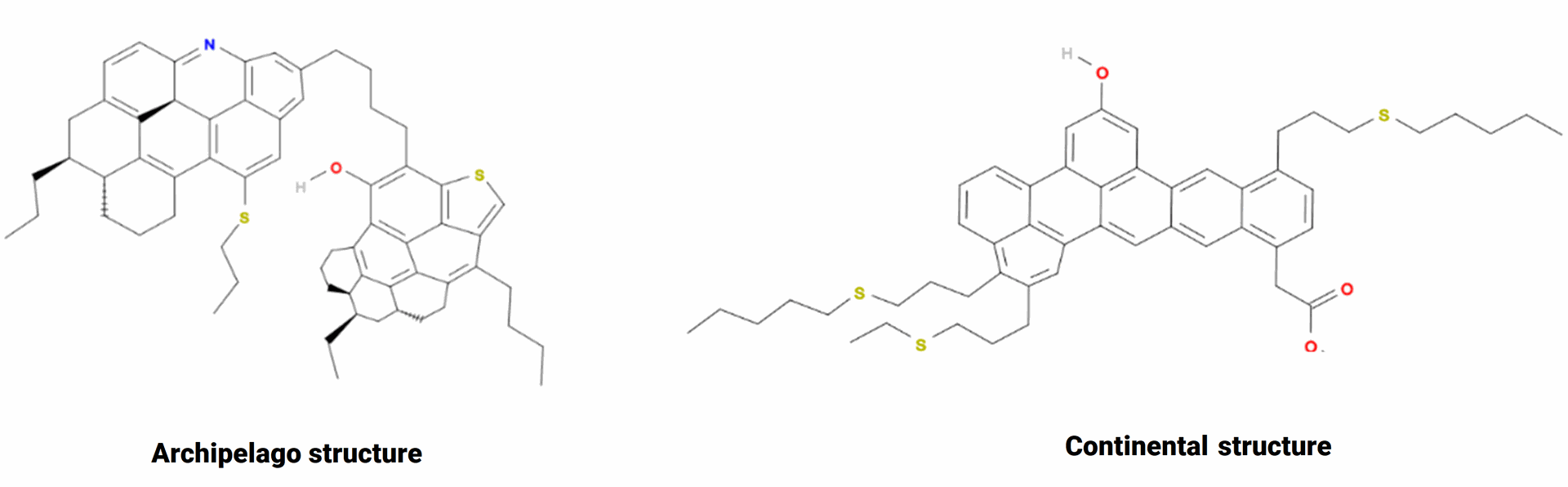
Fig. 2 : Asphaltenes structures : Archipelago (left) Continental structure (right) source (
Ahmadi, M.; Chen, Z.
Symmetry 2020, 12, 1767. https://doi.org/10.3390/sym12111767
)
Asphaltene fractions were found to show different composition according to their carbonaceous source. For example petroleum derived asphaltenes are different in composition from coal derived asphaltenes.
Curiously, asphaltenes from the same source can exhibit different properties and compositions depending on their method of extraction. For instance, pentane-induced asphaltenes are different from CO2-induced asphaltenes, and both are different from depressurization-induced asphaltenes. Interestingly, asphaltenes extracted with different alkanes can also show different features and compositions (as in Fig.3), so it is very typical to see asphaltenes designated by the solvent used to extract them. Thus, C5 asphaltenes are those extracted by pentane, C7 asphaltenes are the ones extracted by heptane, and so on. Given the above observations, it is expected that lab generated asphaltene deposits will differ from field formed asphaltene deposits, which have been reported in many studies.

Fig.3: Examples of appearance of asphaltenes separated from the same crude oil with different alkane precipitant , n-heptane (right) , n-pentane(left) source ( NMT ASPHALTENE FAQ , New Mexico Tech. n.d., What are asphaltenes?. Petroleum Recovery Research Center )
Well, it is a little obvious now why asphaltenes are somehow confusing; it is because of their complex composition and the varying yields obtained during extracting them for lab investigations. As a result, you might find asphaltenes behave differently in aqueous solutions compared to organic solvents; they even behave differently at different dilutions of organic solvents.
Therefore, it's not surprising to find confusing or even conflicting reports on asphaltene's different properties, such as molecular weight, charge, and others. It is also worth mentioning that there is a long list of various techniques that have been used to study asphaltenes and other heavy fractions, such as mass spectrometry, electron microscopy, nuclear magnetic resonance, small-angle neutron and x-ray scattering, ultrasonic spectroscopy, dynamic light scattering, fluorescence correlation spectroscopy, fluorescence depolarization, vapor-pressure osmometry, gel permeation chromatography, and others. Thus, it is expected that different techniques will give relatively different results.
According to the recent view of asphaltenes by the Yen-Mullins model, asphaltenes are dispersed or suspended in crude oils and/or in solvents in three forms: molecules, nanoaggregates, and clusters of nanoaggregates, as depicted in Fig.4.
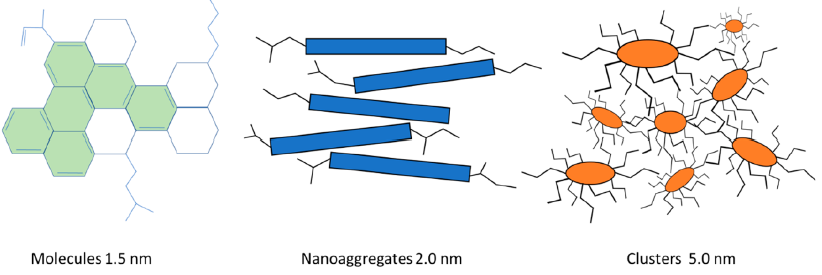
Fig.4: Asphaltene structures according to a modified Yen−Mullins model.(source : Boczkaj et al. Ind. Eng. Chem. Res. 2023, 62, 2−15 https://doi.org/10.1021/acs.iecr.2c02532)
Asphaltene stability and their mechanisms of precipitation have been the subject of continuous investigation over many years. Two major theories have been proposed to explain asphaltenes stability and aggregation:
The first one is based on "colloidal models" where asphaltenes are stabilized by the resins adsorbed on the asphaltene surface; the disturbance of the asphaltene-resin complex/balance causes the asphaltene precipitation (Fig.5). According to this scenario, asphaltenes precipitation is an irreversible mechanism.
The second one is based on "solubility models," in which asphaltenes are assumed to be a solute dissolved in crude oil, where asphaltenes precipitation can be treated as liquid-liquid or solid-liquid equilibria. In this approach, asphaltenes precipitation is a reversible thermodynamic process.
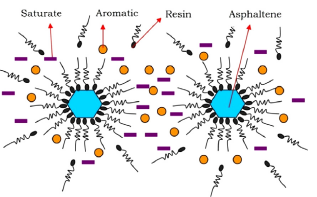 Fig.5: The colloidal model (source: Ashoori et al. 2017 Egyptian Journal of Petroleum Volume 26, Issue 1, March 2017, Pages 209-213)
Fig.5: The colloidal model (source: Ashoori et al. 2017 Egyptian Journal of Petroleum Volume 26, Issue 1, March 2017, Pages 209-213)
When conditions change, asphaltenes can be destabilized and start to aggregate and undergo a hierarchical process of precipitation from asphaltene molecules to end up with fully aged asphaltenes deposits (Fig.6).
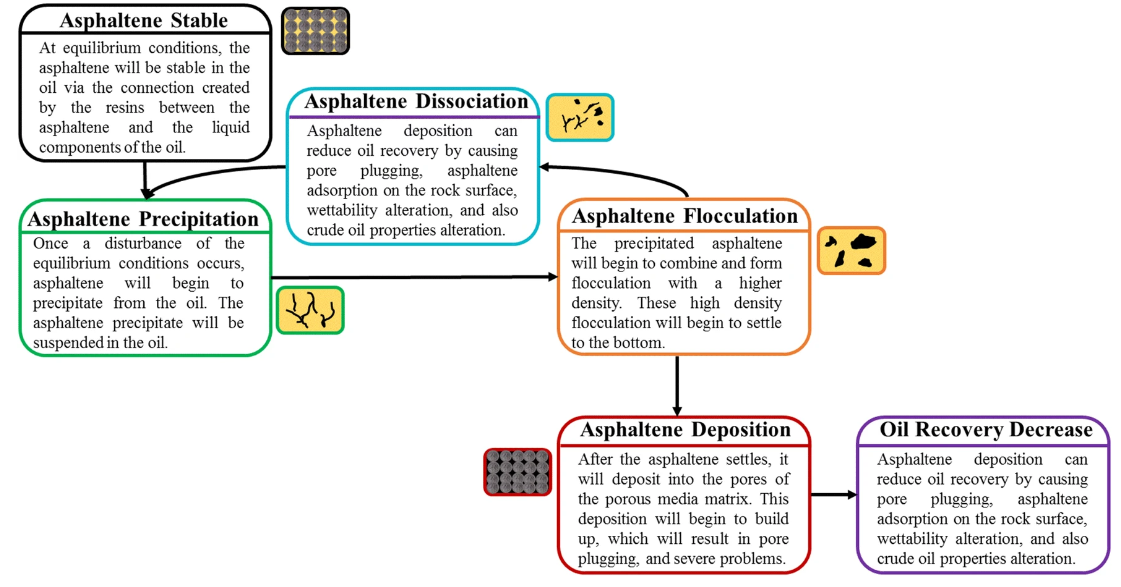 Fig.6: Asphaltene life cycle (source:
Fakher et al 2020,J Petrol Explor Prod Technol 10, 1183–1200 (2020). https://doi.org/10.1007/s13202-019-00811-5)
Fig.6: Asphaltene life cycle (source:
Fakher et al 2020,J Petrol Explor Prod Technol 10, 1183–1200 (2020). https://doi.org/10.1007/s13202-019-00811-5)
Here comes the big question: what makes asphaltenes aggregate and deposit?
Well, it's anything and everything! And this is not an exaggeration, but a fact concerning asphaltenes, and that’s what makes them a challenging flow assurance issue.
Examples of the factors that affect asphaltenes precipitation include:
- Pressure drop, especially near bubble point
- Temperature change
- pH shift
- Mixing different incompatible crudes
- Adding alkanes or incompatible solvents
- High metal ions content
- Corrosion
- Acidizing and acid cleaning
- CO2 injection
- Gas lift operations
- Hydrodynamics /shear
- Streaming potential
Therefore, in order to effectively manage asphaltenes problems in a production system, it may be necessary to carry out a thorough system analysis in order to identify the true cause of their destabilization.
Further reading:
- Hussein (2023), Essentials of Flow Assurance Solids in Oil and Gas Operations, Elsevier.
Asphaltenes (1): The Elusive Danger!