20 October 2023| By Abdullah Hussein
Barium sulfate (BaSO4) (barite) is one of the most challenging types of scale that commonly forms in oilfields.
Due to its properties and its harsh impacts on production, barite can be a nightmare for operators if it started to build up in their production system. Generally speaking, barite is known for:
- Its very low solubility ( 2.3 mg/L)
- Its high mechanical strength
- Its rapid formation and accumulation rates. In the North Sea, a well went from 30,000 BPD to zero BPD in just 24 hours due to barite scale formation.
- It frequently accumulate radioactive materials with it , forming what is known as NORM scale.

Fig.1: Scale sample comprises mainly BaSO4, SrSO4 , the sample also was radioactive.
Sometimes the chemical removal methods of barite fail, and instead mechanical methods are used. On other occasions, neither method works and the operators end up cutting or replacing the scaled part out of the system.
Some BaSO4 dissolvers are available commercially, with some barriers including the cost of these chemicals (especially when huge amounts are required), and the dissolution kinetics and conditions (some dissolvers are slow acting, need high temperature, etc).
A good alternative is the chelating agents known as Polyaminocarboxyates (PAC) such as EDTA, DTPA, CDTA, NTA (Fig. 2) which have been successfully used as BaSO4 dissolvers in oil and gas fields.
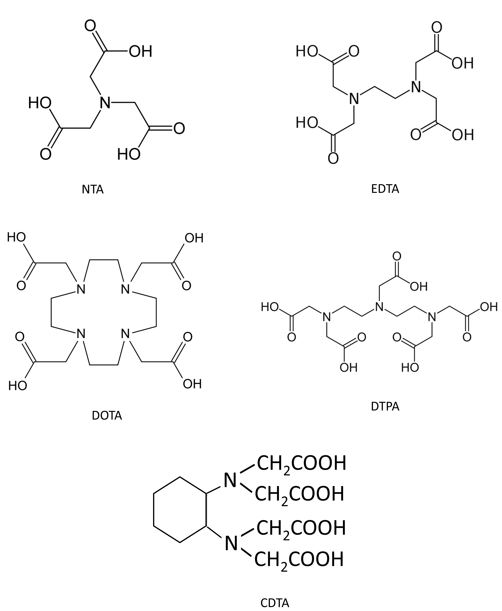
These chemicals have been used for different applications in oil and gas fields, but as scale dissolvers, they are considered :
Cheap.
Non-corrosive.
Work as scale dissolvers for other types of scale such as : BaSO4, CaCO3, CaSO4, and some sulfide scales.
Easy to handle, with less safety and environmental issues.
Easy to formulate and improve their efficiency using different catalysts.
While the literature has shown variable efficiency in using PACs as scale dissolvers, their efficiency essentially depends on how you apply them to get the best of their performance. Here are some helpful tips to effectively remove barite scale using PAC at a cheap price:
- Lab analysis is used to determine the scale deposit's full composition (compounds, polymorphs). Layer-wise composition is also recommended. Physical properties such as scale thickness, and hardness are crucial information for the cleaning job design.
- DTPA is considered the most effective PAC in dissolving barite, but it is more expensive than the others. So, lab dissolution studies should be conducted with various PACs since EDTA might be sufficiently enough to do the job at cheaper price.
- Lab dissolution studies (Fig.3) are conducted to determine: effective concentration, use of catalysts, best temperature, soaking time, and side effects, if any.
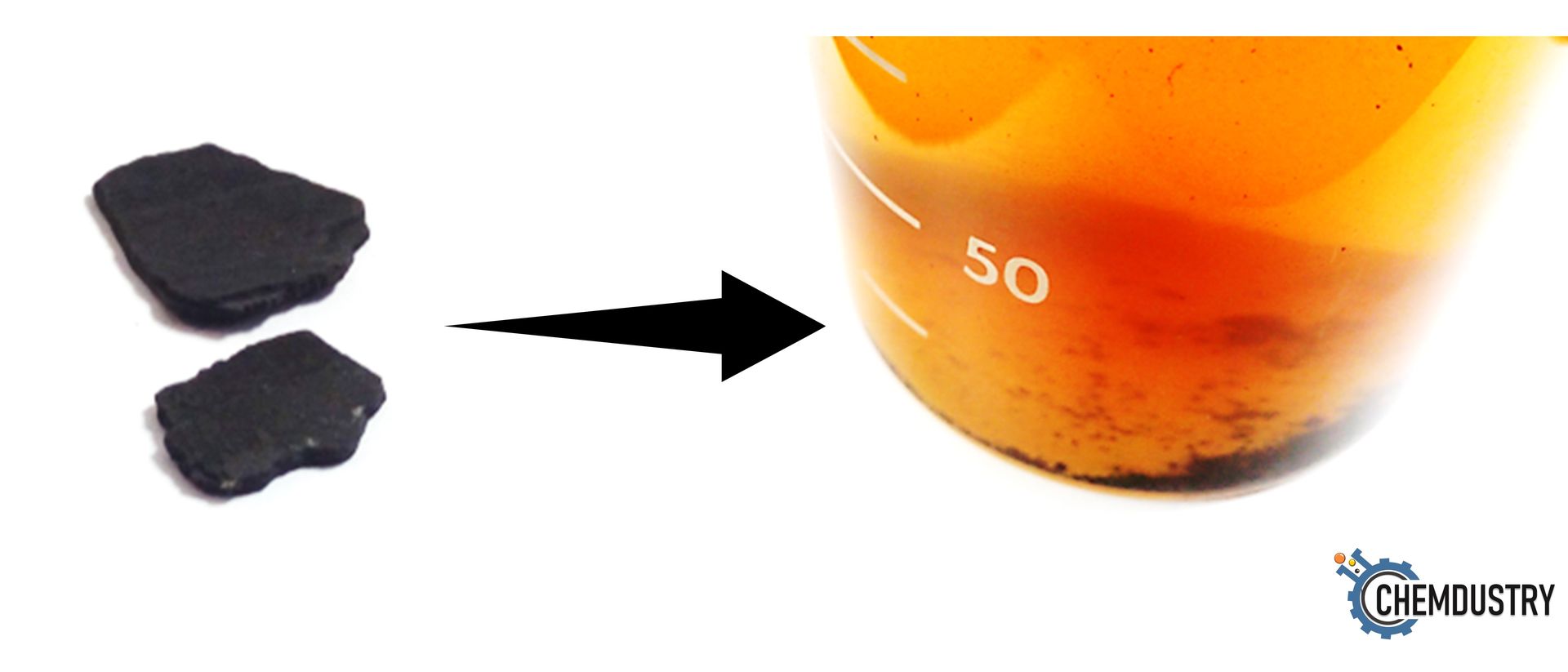 Fig. 3: Dissolution of barite scale sample using PACs
Fig. 3: Dissolution of barite scale sample using PACs
- Various catalysts can be used to boost PACs performance such as: oxalate, thiosulfate, glycolate, maleate, succinate, even phosphonate can be as catalysts.
- pH has to be >10 (~ 10-11.5) to get the best performance of PACs. The capacity of PACs as chelating agents increases with increasing pH, but at pH> 11.5 some precipitations might occur in contact with the produced water.
- High temperature is recommended (> 60 oC). The high temperature of the downhole is utilized, while heaters or heat exchangers might be used as the source of heat during cleaning surface facilities.
- Sufficient soaking time is necessary. It might take hours to a few days to dissolve a thick, dense barite scale layer. Amorphous scale should be easier to dissolve in a shorter time.
- Start the cleaning with organic solvent flushing to remove the oil film or organic deposits that might be covering the barite scale.
- If the scale sample analysis showed the existence of acid soluble components, then you can perform inhibited acid flushing to dissolve these components at first. This step will make the scale layer amorphous and decrease its strength significantly specially if these acid soluble components are like cementing materials in the scale layers. A water flush might be necessary after the acid flush to avoid acid residue interactions with the high pH PACs main treatment.
- Soak the dissolver for the predetermined time. Depending on the scale thickness and age, a second or maybe third soaking might be necessary.
- Agitation or circulation is absolutely recommended (if possible)
- Mechanical aids help improving the dissolution efficiency. Wireline scratchers and cutters can be implemented to improve the dissolution of scale. In surface operations, pigging can be implemented to improve the dissolver efficiency. Coiled tubing can be applied in downhole and topside facilities and combine the use of the mechanical power, the chemical dissolver power and the benefit of heat.
--------------------------------------------------------------
Further reading
Chemical removal of barium sulfate scale