15 September 2023 | By Abdullah Hussein
Two different classes of cage-like molecules are commonly encountered in oil and gas fields, particularly in gas handling systems: gas hydrates and diamondoids.
Gas hydrates are considered a flow assurance issue and a safety hazard. They are non-stoichiometric solid crystals that form when guest gases entrapped in molecular cages of water (Fig.1).
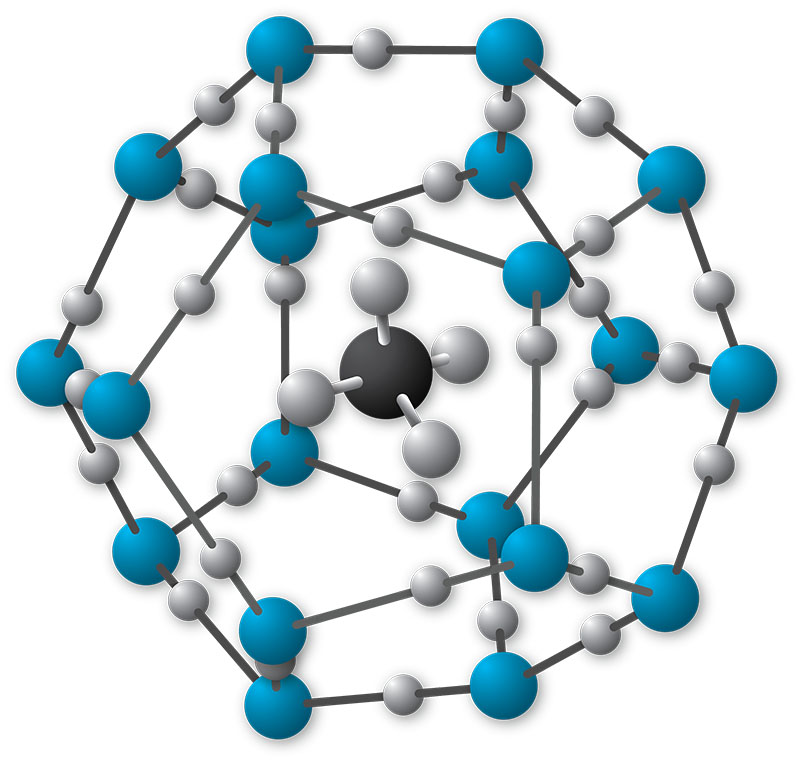 Fig.1: Structure of methane hydrate (
Image courtesy of NOAA Ocean Exploration)
Fig.1: Structure of methane hydrate (
Image courtesy of NOAA Ocean Exploration)
Gas hydrates exist in different structures, most commonly the cubic structures sI and sII and the hexagonal structure sH (Fig.2).
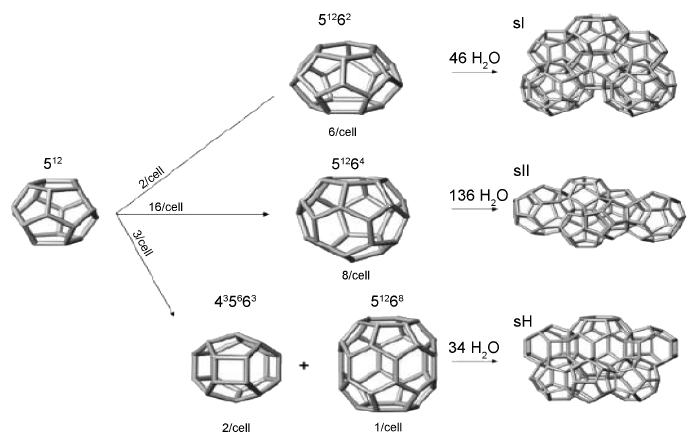
Fig.2: Structures of gas hydrates
(Source : Aregba, (2017) Open Journal of Yangtze Gas and Oil , 2, 27-44.)
The general conditions under which gas hydrates can form include:
- Natural gas at or below its dew point normally with free water present
- Low temperature (at or below the hydrate formation temperature-HFT)
- High pressure (at or above the hydrate formation pressure-HFP)
- Sufficient agitation
- Presence of seed crystals of hydrates
- If acid gases H2S or CO2 are present, they can enhance the gas hydrate formation
- If surfactants are present, they will enhance gas hydrate formation
- Hydrophobic surfaces also enhance gas hydrate formation
Gas hydrate is the primary threat for deepwater drilling and development, and its blockages are the worst to deal with due to the extreme danger during removing them because of their very high expansion ratio e.g. methane hydrate can expand up to 164 folds, a common example usually given is like a man expands into the size of the Eiffel tower.
This danger has been reported to cause fatal incidents and disasters such as the deepwater horizon in the Gulf of Mexico and piper alpha in the North sea.
Gas hydrates management include : sampling the fluids, performing hydrate predictions, and maintain a mitigation strategy either using operational methods (gas dehydration, optimize P, T) chemical methods ( THI, LDHI) or nonchemical methods (cold flow, heating system, insultation, etc).
Diamondoids is another flow assurance issue. They are saturated polycyclic hydrocarbons. They are called diamondoids because they are similar to diamonds in their chemical structure.
They usually deposit when the pressure and temperature of the fluid containing diamondoids decrease, and saturation in the gas is reached, leading to condensation and solidifying a portion of the diamondoid components.
Examples of diamondoids are : Adamantane (C10H16), Diamantane (C14H20), & Triamantane (C18H24) (Fig.3).
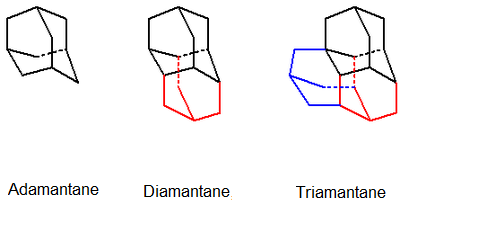 Fig.3: Structures of some diamondoids (source: Wikipedia, courtesy of
V8rik)
Fig.3: Structures of some diamondoids (source: Wikipedia, courtesy of
V8rik)
Although little research has been done on them from the flow assurance side, they have been studied for different applications due to their exotic properties such as high thermal conductivity, high hardness and chemical resistance.
Gas hydrates and diamondoids