23 March 2024| By : Abdullah Hussein
Sulfur is one of the most abundant elements on earth. Sulfur occurs in various forms as an essential component of several minerals, including the sulfate and sulfide minerals, or it can be found in its pure native form (S8). Sulfur also has many oxidation numbers, most commonly +6, +5, +4, +2, and -2. The circulation of the various sulfur compounds between rocks, waterways, and living systems is represented by a biogeochemical cycle known as the sulfur cycle (Fig. 1).
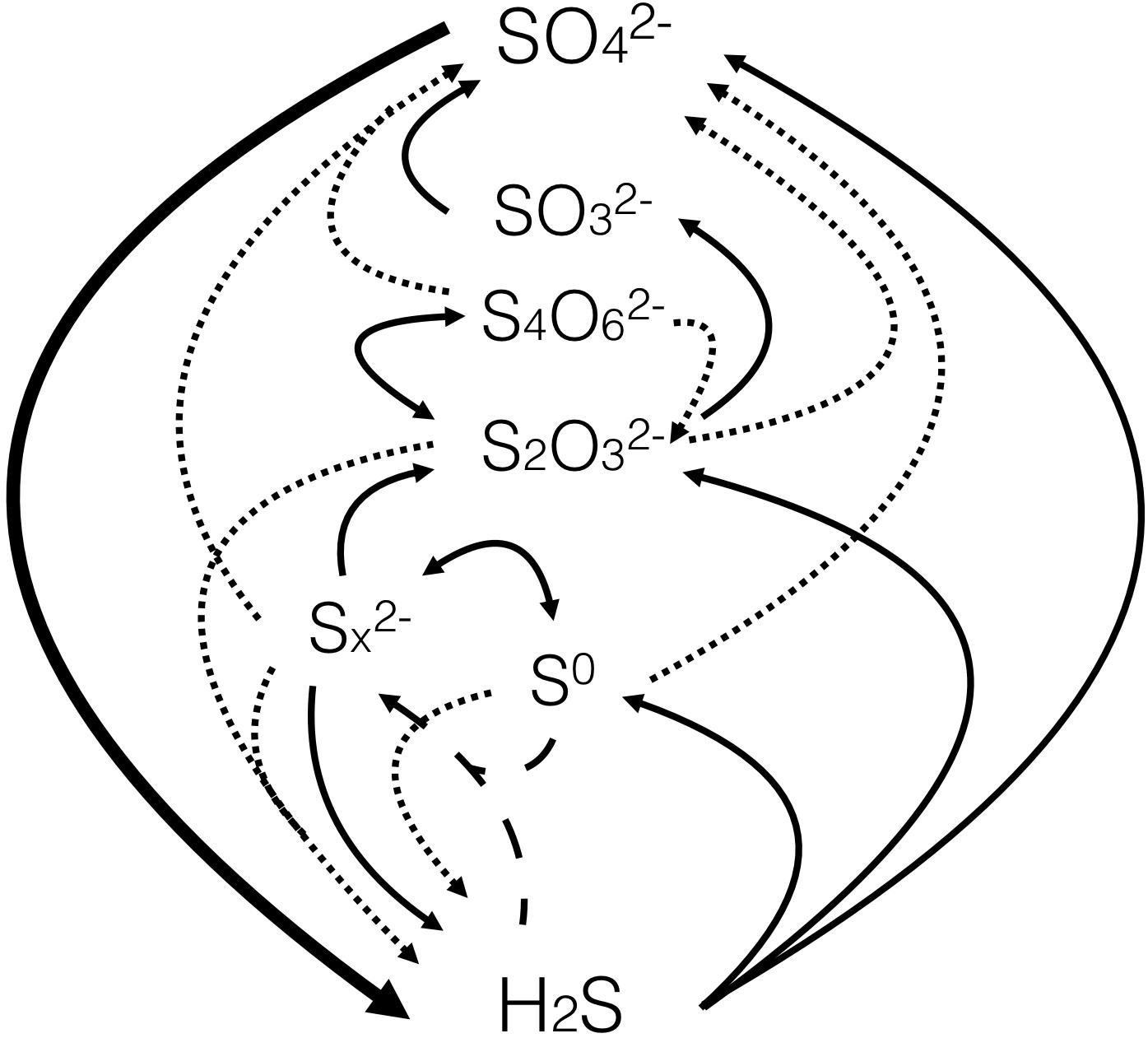
Fig.1 : Major inorganic species in sulfur cycle (source:
Jørgensen BB et al. (2019) The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 10:849. doi: 10.3389/fmicb.2019.00849)
Sulfur species also exist abundantly in petroleum systems, in reservoir rocks, in reservoir and injection waters, and in crude oil components. One of the most common and dangerous sulfur compounds is hydrogen sulfide (H2S). The generation of H2S is one of the biggest challenges in oil and gas fields due to its impacts on field operations and personnel. H2S is colorless, highly flammable gas, explosive, heavier than air, corrosive, smells like rotten eggs at very low concentrations, and at high concentrations can be life-threatening if not handled properly. The structure and physical properties of H2S are shown in Fig. 2.
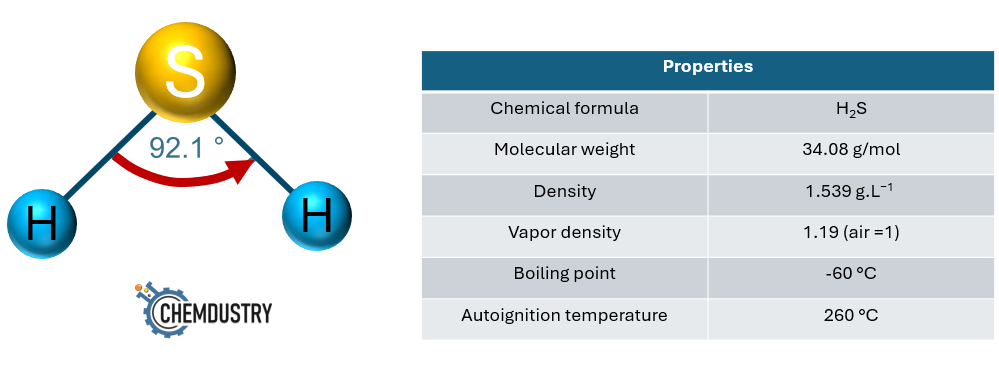 Fig.2.: H2S structure and properties
Fig.2.: H2S structure and properties
Reservoir souring is the process when H2S is produced within the reservoir. Hydrocarbon reservoirs are called sour reservoirs if the produced gas stream contains more than 3 ppmv of H2S. Production operations face a number of issues as a result of H2S generation and reservoir souring, including:
- Sour corrosion, which severely impacts facilities integrity and causes their rapid deterioration.
- Formation of various sulfide scale deposits, causing flow restrictions and blockages. Sulfide scale can also exaggerate the corrosion problem and stabilize emulsions.
- A considerable increase in production costs as a result of H2S mitigation and its adverse effects.
- When H2S is generated in significant amounts, the operations are deemed unsafe, and that leads to wells shut-in.
- H2S has serious health impacts on personnel, as seen in Fig. 3 below.
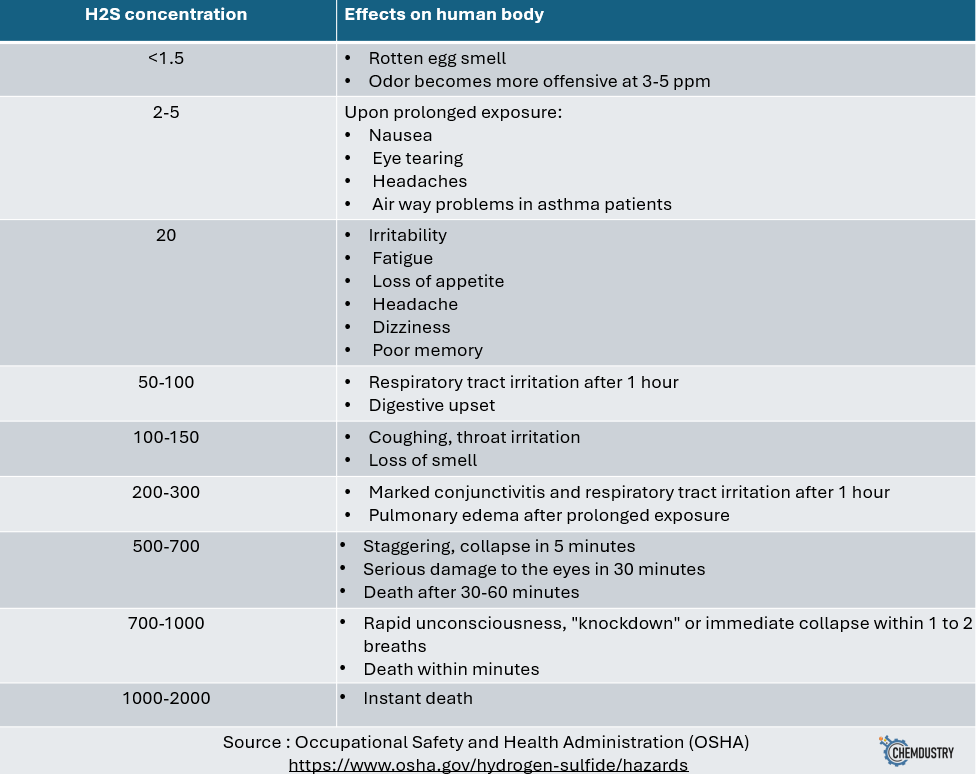
Fig.3: impacts of H2S on humans health
Biotic generation of H2S
Biotic H2S generation usually takes place by sulfate reducing prokaryotes (SRP) or sulfate reducing microorganisms (SRM), through anaerobic sulfate reduction. In this mechanism, sulfate is used as an electron acceptor and a source of energy by SRP during the anaerobic respiration, whereas the electron donor can be a carbon source, hydrogen, or metal pipes. Figure 4 depicts the sulfate reduction mechanism.
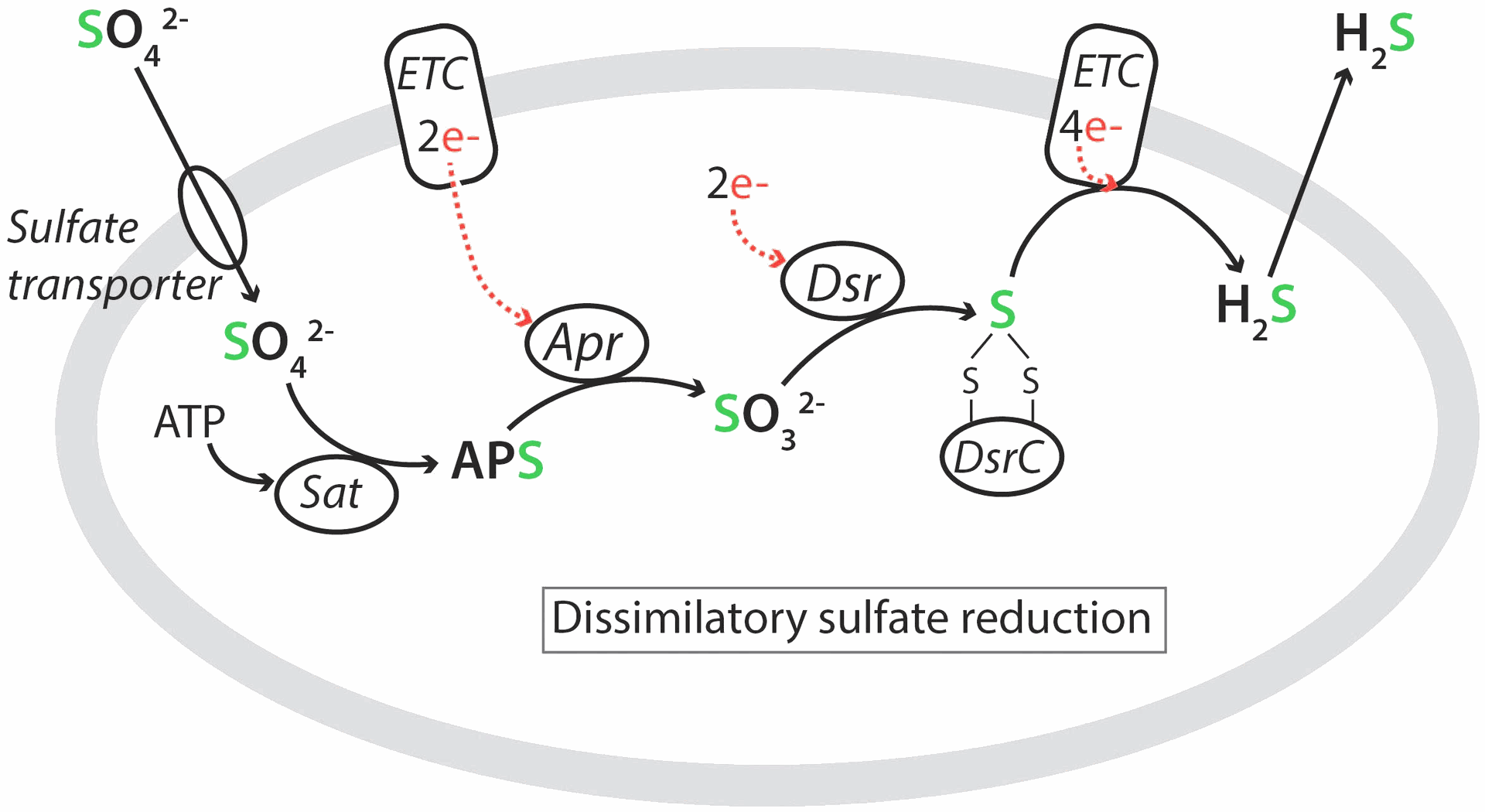 Fig.4 : Dissimilatory sulfate reduction by SRP,
Sat, ATP sulfurylase; APS, adenosine-5′-phosphosulfate; Apr, adenylyl-sulfate reductase; Dsr, dissimilatory (bi)sulfite reductase; ETC, membrane-bound electron transfer complex.
(source:
Jørgensen BB et al. (2019) The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 10:849. doi: 10.3389/fmicb.2019.00849)
Fig.4 : Dissimilatory sulfate reduction by SRP,
Sat, ATP sulfurylase; APS, adenosine-5′-phosphosulfate; Apr, adenylyl-sulfate reductase; Dsr, dissimilatory (bi)sulfite reductase; ETC, membrane-bound electron transfer complex.
(source:
Jørgensen BB et al. (2019) The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 10:849. doi: 10.3389/fmicb.2019.00849)
SRP includes sulfate reducing bacteria (SRB) and sulfate reducing archaea. Desulfovibrionales, Desulfosporosinus, Desulfotomaculum are common genera of SRB found in oil and gas fields.
These SRP can be indigenous to the reservoir or could be introduced to the reservoir during drilling, workover, fracturing, flow assurance mitigation (chemical treatment injected into the reservoir, e.g., scale inhibitor squeeze), or during water flooding operations. Using sea water injection in water flooding is a major source of reservoir souring due to the high sulfate content in sea water (~ 3000 ppm) and the existence of various sulfate-reducing communities.
Abiotic generation of H2S
In this pathway, H2S is generated without the need for microorganisms. H2S can be generated abiotically by various mechanisms, including the following:
- Thermochemical sulfate reduction (TSR)
In this mechanism, sulfate ions can be reduced by various organic compounds at very high temperatures (above 250 °C). The sulfate ions are usually derived from reservoir water, seawater, pore water, or the dissolution of sulfate-bearing minerals in the reservoir (e.g., gypsum and anhydrite). H2S generated by TSR can be in high concentrations (> 10%).

- Thermolysis or aquathermolysis
In this mechanism, organosulfur compounds produce H2S at high temperatures. Aquathermolysis occurs at temperatures below 240 °C, whereas thermolysis takes place at higher temperatures. The organosulfur compounds involved in this reaction include thiophene, tetrahydrothiophene, thiols, and others, as depicted in Fig.5.
The H2S generated by this mechanism is usually < 5%, depending on the sulfur content of the compounds.
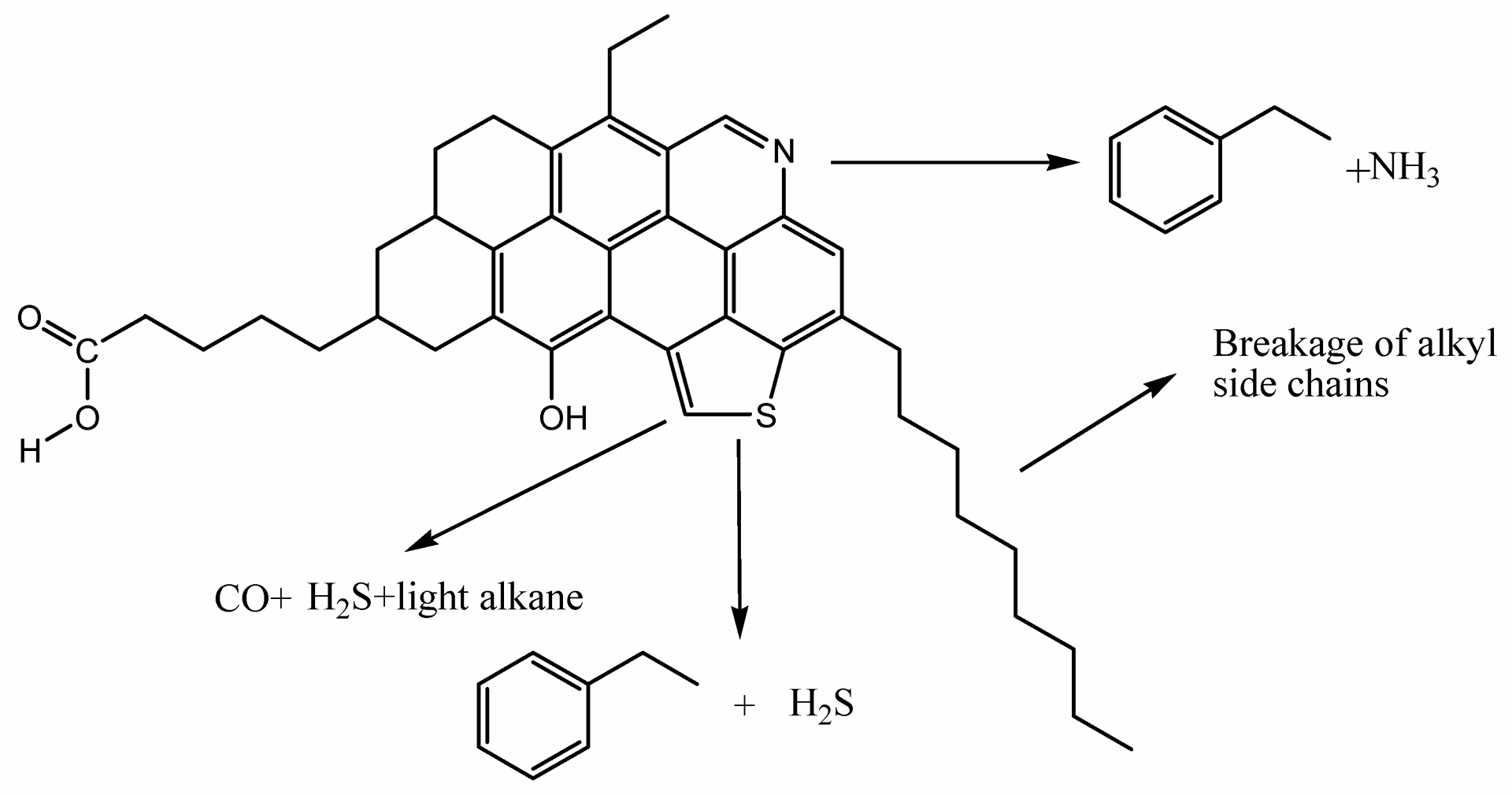
Fig.5: Chemical changes occurring in the aquathermolysis reaction of heavy oil, source :
Zhang W, et al. Catalysts. 2022; 12(11):1383. https://doi.org/10.3390/catal12111383
- Dissolution of sulfide minerals
Minerals such as pyrite, sphalerite, and galena that exist in the reservoir can dissolve under acidic conditions to produce H2S. The dissolution can take place during water injection, steam injection, or by the effect of the injected acidic chemicals, such as stimulation chemicals, biocides, scale inhibitors, and other chemicals that are introduced to the formation.
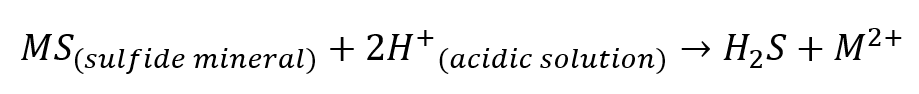
Hydrogen Sulfide in Petroleum Fields (1): An Introduction