15 December 2023 | By Abdullah Hussein
Mineral scales deposition is a major flow assurance problem in oil and gas operations. Scale formation imposes several challenges on production operations, such as formation damage, flow restrictions and blockages, expensive maintenance, and production downtime.
scale formation mechanism is explained in a few basic steps, including:
- Supersaturation
- Nucleation
- Crystal growth
- Adhesion
- Aging
If we look at the scale mitigation methods (chemical or nonchemical), we find they rely essentially on interfering with one or more of these formation steps, which ultimately prevents scale formation or at least attenuates their effects (Fig.1).
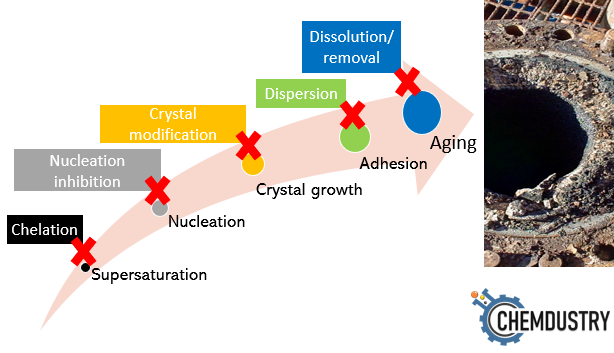
Fig.1: Scale formation steps and their counter mitigation steps
■ Chemical methods are based on using scale inhibitors, scale dispersants, and scale dissolvers. Scale control chemicals act in many different ways that interfere with the scale formation steps, as follows:
- Chelation (interferes with supersaturation)
The chelating agent (chelants) binds with the metal ions in the solution, preventing them from interacting with the counter ions and forming a precipitate [Fig.2]. Chelants are added in a stoichiometric ratio with the scaling metal ions. Common chelating agents : polyaminocarboxylate.
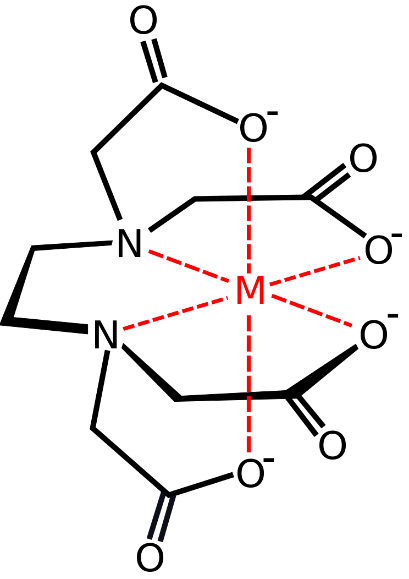
Fig.2: EDTA chelation with metal ions (source: wikipedia)
- Nucleation inhibition/sequestering (interferes with nucleation)
Scale inhibitors adsorb on the formed nuclei, prevent them from further growing, and keep them at submicroscopic size. These chemicals are added in very low concentrations (few ppm), so they are known as threshold inhibitors.
- Crystal modification (interferes with crystal growth)
Scale inhibitors adsorb on the formed crystal surface (covering 3-5 % of the crystal surface) and cause morphological changes to the lattice, which prevent their further growth or slow it down (Fig.3). These chemicals are also threshold inhibitors.
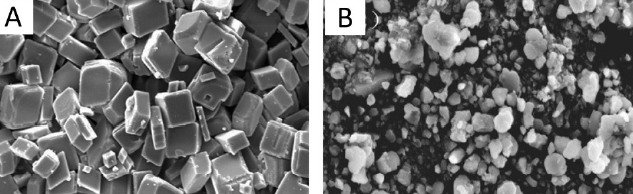
Fig.3: (A) CaCO3 scale crystals without scale inhibitors (B) modified CaCO3 crystals in presence of scale inhibitor. source Zuo et al. 2020, Crystals. 2020; 10(5):406.
- Dispersion (interferes with adhesion )
In this category, scale inhibitors prevent scale formation by dispersing scale particles in the solution via electrostatic repulsion, or by a film-forming mechanism, and they can be added at low concentrations.
Scale inhibitors such as phosphonates, polycarboxylate, polysulfonate, and others are common threshold inhibitors that are known to work with one or more of the above-mentioned mechanisms.
- Dissolution (interferes with the formed /aged scale layer)
Here, the chemical is used to dissolve and remove the formed scale layer. Examples are acids, polyaminocarboxylates, biocides, and others.
■ Examples of nonchemical methods include internal coating and physical methods.
- Internal coatings prevent scale by interfering with the adhesion step which keeps scale particles dispersed in the solution.
- Physical methods such as: ultrasonic, magnetic, electromagnetic, and other methods where a physical signal presumably affects the metal surface, water properties, or scale crystal which ultimately keep scale particles dispersed (Fig.4).
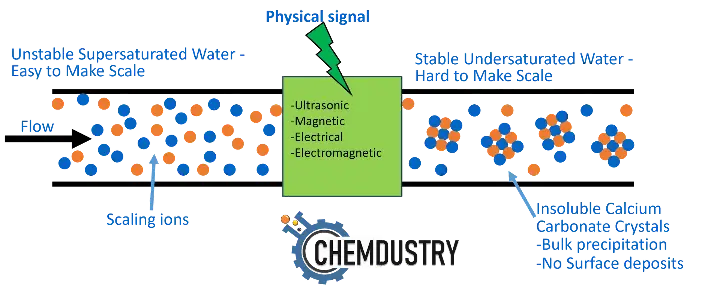 Fig.4: Basics of nonchemical scale inhibition methods
Fig.4: Basics of nonchemical scale inhibition methods
Mineral Scale mitigation methods